Am I Eligible for a Clinical Trial?
DECEMBER 01
Participating in a clinical trial is a significant step in your healthcare journey, offering access to innovative treatments while contributing to groundbreaking medical research. For caregivers, it’s also an opportunity to advocate for loved ones and explore options that could bring hope when traditional treatments fall short. However, not everyone can join every trial. Each clinical study has specific eligibility criteria designed to protect participants and ensure that the research produces meaningful results. Understanding these criteria is a crucial first step in determining whether a clinical trial is a good fit for you or your loved one. In this article, we’ll explore the essential aspects of eligibility, how it ties into trial design, and how platforms like PatLynk can simplify the process of finding a suitable match. If you’re considering a clinical trial, our guide on How to Participate in a Clinical Trial explains the next steps.
By Maria Garzon

Eligibility criteria describe who can and cannot participate in a clinical trial. These guidelines are not meant to exclude individuals unfairly but to ensure the safety of participants and the reliability of the study. Researchers use these criteria to recruit a group of participants with similar characteristics, which helps them understand how a treatment affects a specific population.
An important factor that influences eligibility is the type of study—whether it's interventional or observational.
- Interventional Studies: These trials involve actively administering treatments or interventions to participants to assess their effects on health outcomes. Because these studies test new drugs, procedures, or therapies, they often have stricter eligibility criteria. This is to ensure participant safety and to create a controlled environment where the effects of the intervention can be accurately measured. Participants might need to meet specific health conditions, have certain genetic markers, or be within a particular age range. For example, a trial testing a new medication for Type 2 diabetes might require participants to have had the condition for less than five years and not be on insulin therapy.
- Observational Studies: In these studies, researchers observe participants and collect data without assigning any specific intervention. The eligibility criteria are generally broader to include a more diverse population. This inclusivity helps in understanding how diseases affect different groups in real-world settings. For instance, an observational study on the long-term effects of air pollution might include participants of all ages, backgrounds, and health statuses.
Understanding whether a trial is interventional or observational can help you determine the likelihood of meeting its eligibility criteria. It also provides insight into what participation might involve, whether that's receiving a new treatment or simply providing information over time.
Factors That Affect Eligibility
These categories mentioned above also include different trial subtypes, like treatment, prevention, diagnostic, and quality of life studies, that each with unique goals and eligibility criteria.
For example:
- Treatment Trials: Focus on testing new therapies for specific conditions. Eligibility often considers the stage of the disease, previous treatments, and overall health.
- Prevention Trials: Aim to stop diseases before they occur. Participants might be selected based on family history, lifestyle, or other risk factors.
- Diagnostic or Screening Trials: Assess new ways to detect diseases. They may require participants with specific symptoms or test results.
- Quality of Life Trials: Designed to improve comfort for individuals with chronic conditions. Selection might be based on ongoing treatments and symptoms.
Eligibility criteria can also depend on the trial's phase:
- Phase 1 trials usually have minimal restrictions, focusing on safety in small groups.
- Phase 2 trials require more specific disease-related characteristics to test effectiveness.
- Phase 3 trials involve larger groups and stricter criteria to confirm a treatment's efficacy
- Phase 4 trials—conducted after a treatment is approved—have broader criteria to observe long-term effects.
And they normally assess the following criteria:
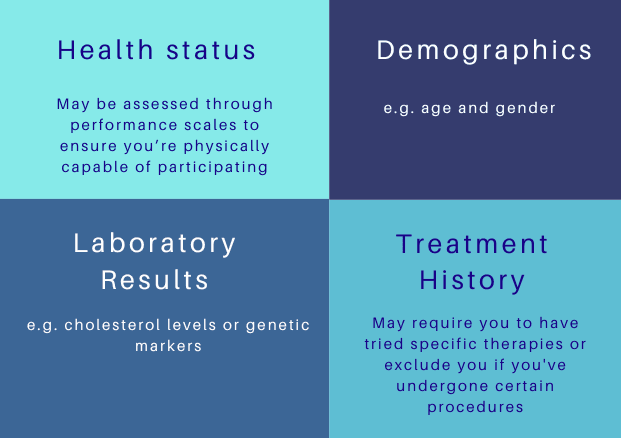
How PatLynk Simplifies the Process
PatLynk streamlines the experience by:
- Automatically matching your health profile to trials that suit your specific needs, saving you time and effort.
- Simplify complex eligibility criteria and medical language into easy-to-understand language.
- Connecting you directly with study teams for clarification or to take the next steps.
For patients, this means clarity and confidence in finding a trial that aligns with your health journey. For caregivers, PatLynk alleviates the stress of navigating the system, letting you focus on supporting your loved ones.
By tailoring the process to individual needs, PatLynk ensures that everyone involved—patients and caregivers alike—can take an active, informed role in advancing health outcomes.
Deciding When to Explore a Clinical Trial
Clinical trials can be an option at different stages of an illness. For some, they offer hope when standard treatments are no longer effective. For others, they may represent an opportunity to access cutting-edge therapies earlier in their healthcare journey. Whether you’re considering a trial for advanced cancer, a rare disease, or a chronic condition, the timing often depends on the patient’s overall health, the stage of the disease, and available standard treatments.
Next Steps
Eligibility is just one piece of the puzzle. Once you’ve identified a potential trial, the process involves confirming eligibility through pre-screening and informed consent. Our guide on How to Join a Clinical Trial explains these steps in detail.
Platforms like PatLynk make navigating the clinical trial landscape more accessible by helping caregivers and patients find trials that match their unique circumstances. This ensures that no opportunity is missed, and every decision is well-informed. Whether you’re a patient or a caregiver, clinical trials can be a path to hope, innovation, and meaningful contributions to medical research.


