Why Participate in Clinical Research?
DECEMBER 01
Clinical trials play an indispensable role in advancing medicine, offering participants the chance to be part of groundbreaking research while potentially benefiting from new therapies. However, deciding to join a clinical trial is deeply personal, and understanding the various reasons people choose to participate can help you evaluate if it’s the right path for you or your loved ones. In recent years, public willingness to participate in clinical research has grown significantly. According to a comprehensive survey conducted by CISCRP, individuals cite a variety of motivations, from supporting medical advancements to accessing new treatment options. This growing interest underscores the need to understand not only the potential benefits of participation but also the considerations and risks involved. Understanding the reasons people participate and how these motivations differ between interventional and observational studies can help you evaluate if clinical research is the right path for you or your loved ones.
By Maria Garzon

Clinical trials play an indispensable role in advancing medicine, offering participants the chance to be part of groundbreaking research while potentially benefiting from new therapies. However, deciding to join a clinical trial is deeply personal, and understanding the various reasons people choose to participate can help you evaluate if it’s the right path for you or your loved ones.
In recent years, public willingness to participate in clinical research has grown significantly. According to a comprehensive survey conducted by CISCRP, individuals cite a variety of motivations, from supporting medical advancements to accessing new treatment options. This growing interest underscores the need to understand not only the potential benefits of participation but also the considerations and risks involved.
Understanding the reasons people participate and how these motivations differ between interventional and observational studies can help you evaluate if clinical research is the right path for you or your loved ones.
Types of Participation: Interventional vs. Observational Studies
Your choice to participate in a clinical trial should align with your goals and expectations, as interventional and observational studies differ significantly in purpose and involvement.
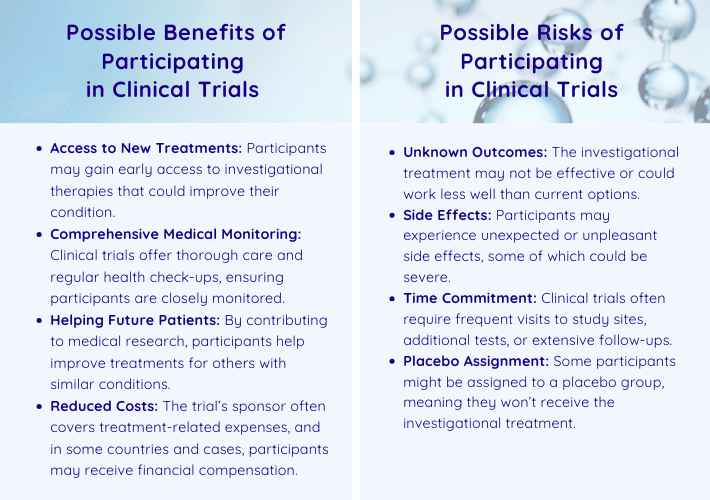
Interventional studies are designed to test new treatments, drugs, or procedures. Participants in these trials may receive an investigational therapy or device as researchers assess its safety, efficacy, and potential benefits. This type of trial is ideal for individuals seeking potential new treatments or therapies, particularly those interested in trying innovative medical interventions that could improve their condition. The benefits of interventional studies include access to cutting-edge treatments not yet available to the public and close medical monitoring throughout the study. However, participants should be aware of potential side effects from experimental treatments and the demanding nature of strict protocols, which may require frequent visits and tests.
In contrast, observational studies focus on monitoring participants in real-world settings without introducing new treatments. These studies aim to uncover patterns, correlations, or risk factors by collecting data while participants continue their regular care. Observational studies are an excellent choice for those whose goal is to support medical research without altering their current treatment. The benefits of these trials include minimal risks—since no experimental treatments are administered—and fewer study visits or requirements compared to interventional trials. While the risks are generally low, participants may need to share sensitive health or lifestyle information, which could be a consideration for some.
Understanding the distinctions between these two types of studies can help you make an informed decision about which aligns best with your goals, whether it’s accessing innovative treatments or contributing valuable data to advance medical research.
Reasons People Take Part in Clinical Research
Let’s delve into the key motivations revealed by the survey conducted by CISCRP, which can be grouped into six main categories:
1. Advancing Medical Science
For many, the idea of contributing to the future of medicine is deeply fulfilling. Participants understand that their involvement can lead to the development of new treatments, diagnostic tools, or preventative measures that may one day save lives or improve the quality of life for countless others. This is especially true for those joining observational studies, where the primary goal is to gather data and insights that could improve care or guide future treatments.
2. Helping Others
Closely linked to advancing science, 43% of respondents expressed a desire to help others who may suffer from their condition. By participating, they contribute to research that could provide relief to patients in the future. This applies to both interventional and observational trials, as each contributes to improved healthcare for future patients.
3. Access to New Treatments
Participants in interventional studies often have the opportunity to access cutting-edge therapies or procedures not yet available to the public. This is particularly appealing for those with conditions that have limited treatment options.
4. Financial Incentives
For 32% of respondents, receiving monetary compensation, which is offered in some counts, was a motivating factor. Financial support may help offset the time, travel, and effort required to participate.
5. Education and Health Awareness
For some, participating in a clinical trial is an opportunity to learn more about their health or condition. It allows them to better understand their treatment options and take an active role in their healthcare journey.
6. Influence of Information
The information read or heard about a study also played a role, influencing 24% of respondents to participate. This highlights the importance of clear and transparent communication about clinical trials.
Each clinical trial comes with its own set of benefits and risks. It’s natural to feel uncertain or have questions about joining a clinical trial. That’s why having all the necessary information is essential to making an informed decision about whether participation is right for you.
Making the Decision
Before deciding to participate, it’s important to:
- Ask Questions: Speak with the study team and your doctor to understand all aspects of the trial, including the risks, benefits, and requirements. For interventional trials, ask about the treatment being tested, potential risks and side effects, and how it compares to standard care. For observational studies, inquire about the type of data collected, its purpose, and whether your current treatment will be affected. Additionally, confirm the time commitment, travel requirements, costs, or compensation, and ask about follow-up care or access to results after the trial. These questions ensure you’re fully informed and prepared for participation.
- Evaluate Your Goals: Reflect on what you hope to gain from participating, whether it’s access to a new drug, personal health benefits, contributing to research, or both.
- Know Your Rights: Participation is entirely voluntary. You can leave the trial at any time if you feel it’s not the right fit for you.
Why Participation Matters
Every breakthrough in medicine has been made possible by those who choose to participate in clinical research. Without volunteers, new treatments, vaccines, and diagnostic tools would remain out of reach for millions of patients in need.
If you’re considering joining a clinical trial, know that your involvement is part of a bigger picture—one that contributes to better care, improved outcomes, and a healthier future for all. At PatLynk we are here to support you as you learn more about opportunities and find studies that match your needs. Together, we can advance medicine and create a healthier future for all.


